
Actions are needed to develop artificial intelligence for glaucoma diagnosis and treatment
Glaucoma is one of the most common ocular diseases that cause irreversible vision loss (1). Because glaucoma has no symptoms until the end stage, with severe visual field defects, patients should be screened for early intervention. To reduce the socioeconomic burden of glaucoma, artificial intelligence (AI) systems are urgently needed to diagnose, monitor, and treat glaucoma. Goldmann et al. described the functional requirements for building a patient-centric computerized AI-based system for glaucoma treatment and care ecosystems (2).
There are many obstacles to overcome to develop artificial intelligence for actual clinical practice. This section briefly reviews the efforts required for the development of AI for glaucoma diagnosis and treatment (Figure 1). First, the criteria for glaucoma diagnosis should be standardized. As commented by Goldmann et al. (2), there is currently a lack of a standardized “ground truth” definition of glaucoma. The spectrum of glaucoma is wide (3), and there is a shortage of glaucoma experts worldwide (4). Therefore, the patterns of dealing with glaucoma are slightly different for each expert, and the treatment criteria differ. This problem poses many obstacles to the development and clinical validation of diagnostic devices for glaucoma. For a more accurate performance, it is important to compare and standardize glaucoma diagnostic data at as many centers as possible and train the AI model based on this verified dataset.
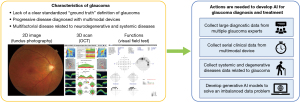
Second, it is important to analyze the time-series and multimodal data of patients with glaucoma. The evaluation of glaucoma commonly involves measuring intraocular pressure, fundus photography, optical coherence tomography, and visual field sensitivity. In addition to the current damage condition, progression of functional or structural damage during follow-up is an important factor in the diagnosis and treatment of glaucoma (5). Each measurement reflects only a few clinical aspects of glaucoma. In addition, errors often occur in one measurement domain; therefore, other domains must be complemented to evaluate glaucoma. Recently, time-series analyses (6) and multimodal deep learning models (7) have been studied for glaucoma diagnosis. In the future, large-scale data analyses based on these approaches will succeed in a more accurate glaucoma evaluation.
Third, detailed data on neurodegenerative and systemic metabolic conditions should be collected along with glaucoma data to predict progression. It is well known that glaucoma is a multifactorial disease associated with metabolic diseases such as diabetes and hypertension (8). In addition, neurodegenerative diseases have been shown to be predictable by fundus photography, and most are closely related to the optic nerve head and the retinal nerve fiber layer in glaucoma (9). AI technology based on multimodal deep learning is increasingly used to analyze high-definition images in every area to reveal the relationship between systemic diseases and retinal images in greater detail (10).
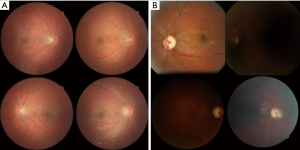
Finally, generative AI techniques should be applied to overcome the lack of pathological data. Data shortages frequently occur because of security or privacy issues. Learning about the imbalanced medical data may result in biased diagnostic model (11). Data augmentation techniques are required for accurate diagnosis in the clinical field, and recently developed generative deep learning models such as generative adversarial networks (GAN) provide solutions to this problem (12). Although still in their infancy (Figure 2), diffusion models, which are newly introduced generation technologies after GAN, can generate fundus photographs (13). As data quality is increasingly improved based on a large amount of data, realistic generative fundus images will be synthesized based on a large amount of data in the future (14).
In conclusion, various strategies are required to develop artificial intelligence for glaucoma diagnosis and treatment. As Goldmann et al. commented (2), this cannot be solved at once and should be based on the interdisciplinary integration and mutual support of all complementary approaches. I believe that this article summarizes all background factors of computer-aided clinical diagnosis, monitoring, and treatment for glaucoma; therefore, researchers interested in this field must check the issues related to AI for glaucoma.
Acknowledgments
The author thanks the Editage for English language editing.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Medical Artificial Intelligence. The article did not undergo external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://jmai.amegroups.com/article/view/10.21037/jmai-23-37/coif). TKY is an employee of B&VIIT Eye Center and VISUWORKS. He received research grants for refractive surgery from Carl Zeiss Meditec AG, personal fees from VUNO, Hangil Eye Hospital and the Korea association of intelligence wellcare industries (KIWI), outside the submitted work. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Soh Z, Yu M, Betzler BK, et al. The Global Extent of Undetected Glaucoma in Adults: A Systematic Review and Meta-analysis. Ophthalmology 2021;128:1393-404. [Crossref] [PubMed]
- Goldmann N, Skalicky SE, Weinreb RN, et al. Defining functional requirements for a patient-centric computerized glaucoma treatment and care ecosystem. J Med Artif Intell 2023;6:3. [Crossref]
- Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA 2014;311:1901-11. [Crossref] [PubMed]
- Li JO, Liu H, Ting DSJ, et al. Digital technology, tele-medicine and artificial intelligence in ophthalmology: A global perspective. Prog Retin Eye Res 2021;82:100900. [Crossref] [PubMed]
- Hu R, Racette L, Chen KS, et al. Functional assessment of glaucoma: Uncovering progression. Surv Ophthalmol 2020;65:639-61. [Crossref] [PubMed]
- Garcia GP, Lavieri MS, Andrews C, et al. Accuracy of Kalman Filtering in Forecasting Visual Field and Intraocular Pressure Trajectory in Patients With Ocular Hypertension. JAMA Ophthalmol 2019;137:1416-23. [Crossref] [PubMed]
- Pham QTM, Han JC, Park DY, et al. Multimodal Deep Learning Model of Predicting Future Visual Field for Glaucoma Patients. IEEE Access 2023;11:19049-58.
- Oh E, Kim YH, Ryu IH, et al. The role of big data analysis in identifying a relationship between glaucoma and diabetes mellitus. Ann Transl Med 2022;10:948. [Crossref] [PubMed]
- Ahn S, Shin J, Song SJ, et al. Neurologic Dysfunction Assessment in Parkinson Disease Based on Fundus Photographs Using Deep Learning. JAMA Ophthalmol 2023;141:234-40. [Crossref] [PubMed]
- Lee YC, Cha J, Shim I, et al. Multimodal deep learning of fundus abnormalities and traditional risk factors for cardiovascular risk prediction. NPJ Digit Med 2023;6:14. [Crossref] [PubMed]
- Wang Shuo, Yao Xin. Multiclass Imbalance Problems: Analysis and Potential Solutions. IEEE Trans Syst Man Cybern B Cybern 2012;42:1119-30. [Crossref] [PubMed]
- You A, Kim JK, Ryu IH, et al. Application of generative adversarial networks (GAN) for ophthalmology image domains: a survey. Eye Vis (Lond) 2022;9:6. [Crossref] [PubMed]
- Kim HK, Ryu IH, Choi JY, et al. Early experience of adopting a generative diffusion model for the synthesis of fundus photograph.
10.21203/rs.3.rs-2183608/v2 [Preprint] 2022 [cited 2023 Apr 23]. Available online: https://doi.org/10.21203/rs.3.rs-2183608/v2 10.21203/rs.3.rs-2183608/v2 - Müller-Franzes G, Niehues JM, Khader F, et al. Diffusion Probabilistic Models beat GANs on Medical Images. arXiv; [Preprint] 2022 [cited 2023 Apr 23]. Available online: http://arxiv.org/abs/2212.07501
Cite this article as: Yoo TK. Actions are needed to develop artificial intelligence for glaucoma diagnosis and treatment. J Med Artif Intell 2023;6:11.


