
Developing a machine learning model using isolated Society of Thoracic Surgeons database variables to predict the presence of clinically-significant ischemic mitral regurgitation
Introduction
The surgical management of ischemic mitral regurgitation (IMR) continues to be a challenging clinical problem with several unresolved clinical controversies. Nonetheless, few would argue that the well-demonstrated adverse impact of persistent IMR on long-term survival supports the addition of concomitant mitral intervention in patients undergoing coronary revascularization who also have moderately severe or severe IMR (1-8). That being said, debate continues regarding both the degree of IMR that warrants concomitant mitral intervention and the choice of mitral repair or replacement as durable therapy (9-15). Further examination and resolution of these important controversial issues using rigorous and innovative investigative methodologies is critical to the evidence-based surgical management of these patients.
In this regard, machine learning offers considerable potential to enhance the current diagnostic and therapeutic clinical algorithms involved in the investigation and surgical care of IMR patients. The unique ability of machine learning algorithms to detect previously unrecognized and counter-intuitive associations between patient-specific clinical variables and the presence of clinically significant disease process, such as IMR, makes their use a promising avenue of clinical investigation (16-18). Further, the ability of these algorithms to identify predictive data patterns in variables drawn from widely disparate sources further enhances their applicability to the modeling of the complex systems active in IMR, since the complexity of the pathophysiology associated with IMR suggests that an equally complex array of multi-source variables may be associated with its occurrence (19,20). The robust, all-inclusive capabilities of current machine learning models allow the combination of variables from multiple clinical sources, thereby maximizing the strength of the models by including a diversity of variable content and source.
The foundation of this complex array of multi-source machine learning variables will likely be comprised of both valvular structural morphology and regional contractile variables (19-27). Nonetheless, patient demographic and other clinical variables (examples might include coronary anatomy, presence of diabetes, or a history of smoking) will almost certainly be contributory to the development of these complex machine learning models. In this regard, the Society of Thoracic Surgeons (STS) Database variables ideally characterize these latter clinical features. Further, their ready availability in almost every cardiac surgical program makes the use of these variables particularly attractive, optimizing the widespread direct clinical applicability of any resulting machine learning predictive models. This investigation is aimed at quantifying the potential for predictive contribution of foundational STS variables by testing the predictive power of their association with the presence of clinically significant IMR in machine learning modeling. The authors present the following article in accordance with the TRIPOD reporting checklist (available at http://dx.doi.org/10.21037/jmai-20-50).
Methods
Source of data
All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Washington University School of Medicine Institutional Review Board (IRB Registration # 201905091). A waiver of informed consent was granted. The STS Adult Cardiac Surgery Database (ACSD) versions 2.30 through 2.9 were queried from January 1996 to December 2018. Baseline demographics, chronic comorbid conditions, imaging (coronary angiographic and echocardiographic) data, and revascularization targets were included.
Participants
Between 1996 and 2018, a total of 7,079 patients underwent surgical myocardial revascularization with or without mitral valve intervention at a single institution, Barnes-Jewish Hospital at Washington University Medical Center in Saint Louis, Missouri. Patients undergoing other concomitant interventions (such as an aortic valve replacement or Cox-Maze procedure) were excluded. Of the remaining patients, 416 underwent combined surgical myocardial revascularization and mitral valve intervention. The operative reports of these 416 patients were reviewed and the study cohort was limited to only those patients in whom an ischemic etiology to the mitral regurgitation was documented. Patients who underwent mitral valve intervention due to any etiology other than IMR (such as endocarditis, rheumatic valvular disease, or myxomatous degeneration) were excluded, as were emergent patients with ruptured papillary muscles. The final study group included a total of 7,005 patients undergoing surgical myocardial revascularization with (n=363) or without (n=6,642) mitral valve intervention for IMR.
Predictors
A total of 53 STS Database variables, all of which could be tracked through the many format changes implemented during the timeframe of the study, were chosen to serve as predictors of need for concomitant mitral valve intervention in patients undergoing surgical myocardial revascularization. These variables included all preoperative STS variables relating to demographics, comorbidities, coronary artery disease architecture, and coronary artery bypass targeted vessels.
Since patients with all other associated valvular and aortic disease interventions (except IMR) were excluded from the study cohort, the STS Database variables related to other valvular and aortic disease were also excluded. Similarly, all postoperative STS Database variables were excluded in this predictive model, as this investigation is targeted at defining the association between preoperative variables and the presence of clinically significant IMR. Further, the STS Database variables specifically involving the presence or absence of mitral valvular disease were excluded. The final set of STS Database variables included in the development of the machine learning models are listed in Table 1.
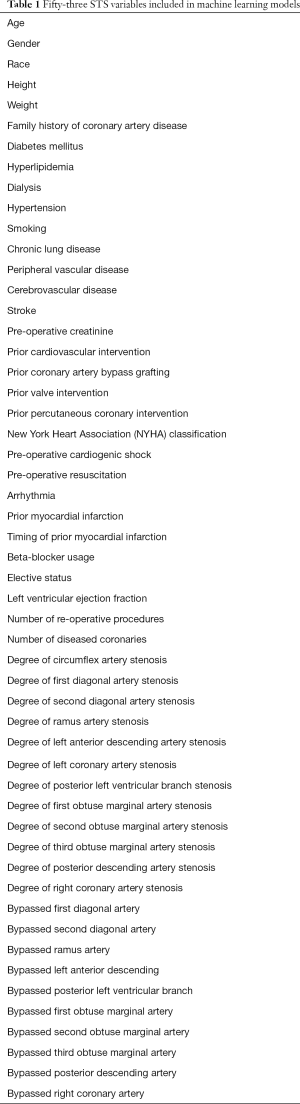
Full table
Outcome
This investigation sought to test the predictive value of foundational STS variables in machine learning algorithm to predict the presence of clinically significant IMR in patients undergoing surgical myocardial revascularization.
Statistical analysis
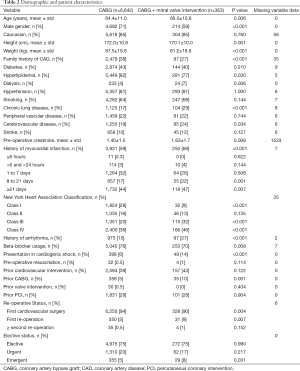
STS variables were compared between patients who underwent coronary artery bypass grafting (CABG) only (n=6,642) and those that underwent CABG plus concomitant mitral valve intervention (n=363). Continuous variables were expressed as mean ± standard deviation, as shown in Table 2. Student’s t-test was used to compare means of normally distributed continuous variables, while Mann-Whitney U test was used for skewed distributions. Categorical variables were compared using either χ2 analysis or Fisher’s Exact test. A P value <0.05 was considered statistically significant.
Full table
Machine learning analysis
Utilizing a standard ML approach that acknowledges the unknowable potential advantages of the various available ML algorithms in any given unique dataset, we employed multiple, widely varying ML and deep learning models. These included random forests (RF), support vector machines (SVM), logistic regression (LR), and deep neural networks (DNN) (28-31). Missing data values were replaced by mean (continuous case) or mode (categorical case) values. The RF, linear SVM, and LR models were configured by the default options in Scikit-learn in Python 3. Specifically, the RF model was configured as follows: the number of trees in the RF n_estimators = 100; the number of features considered when looking for the best split max_features = ‘auto’; the minimum number of samples at a leaf node min_samples_leaf = 1. The SVM model was configured as follows: the regularization parameter C = 1.0; kernel type kernel = ‘rbf’; the kernel coefficient gamma = ‘scale’. The LR model was configured as follows: the norma used in the penalization penalty = ‘l2’; tolerance for stopping criteria tol = 1.0*10 − 4; the unverse of regularization strength C = 1.0; algorithm used in the optimization problem solver = ‘lbfgs’.
Our DNN model was comprised of an input layer (with 53 dimensions), 5 hidden layers (with 256, 256, 128, 64 and 32 dimensions respectively) and a scalar output layer. We used the Sigmoid function at the output layer and ReLu function at each hidden layer (32,33). Binary cross-entropy was used as loss function and the Adam optimizer was employed with a mini-batch size of 64 samples (34). This final model was obtained by the iterative testing of multiple variations using different numbers of nodal dimensions, as well as multiple different nodal activation functions, loss functions, and optimizers. The final model resulted in the best DNN predictive accuracy.
All machine learning analyses were carried out using Python 3 (www.python.org) programming language. Data was prepared and managed using Scikit-learn (https://scikit-learn.org), NumPy (https://numpy.org), pandas (https://pandas.pydata.org), TensorFlow (https://www.tensorflow.org), and Keras (https://keras.io). All analyses were carried out on laptop computers using only standard CPU’s.
The supervised machine learning using binary classification that was employed in this investigation was based solely upon the presence or absence of clinically significant IMR. The sole determinant of the “clinical significance” of each patient’s IMR was the operative surgeon’s decision regarding the clinical necessity of adding a concomitant mitral valve intervention to the coronary revascularization. It is recognized that many different patient-specific factors may influence this decision and that the impact of these influences may vary between surgeons. Nonetheless, it is our belief that for each individual patient, the operative surgeon was the most informed judge of the clinical significance of the degree of IMR. They are, therefore, the optimal resource—as reflected by their choice for or against the addition of mitral intervention—for our determination of the presence or absence of clinically significant IMR.
The feature importance analysis indicates feature value in the construction of Pearson’s correlation coefficients within the model. The coefficient value of the feature determines its importance in the accurate prediction of the presence or absence of clinically significant IMR.
In our final model, the positive class included 363 patients who underwent CABG and concomitant mitral valve intervention. The negative class included 6,642 patients who underwent isolated coronary revascularization. The data were randomly partitioned into training (80%) and testing (20%) sets. The training set had 5,604 total patients, with 281 from the positive class and 5,323 from the negative. The test set had 1,401 total patients with 82 from the positive class and 1,319 from the negative. Due to the imbalance in the classes in the data set, the Synthetic Minority Oversampling Technique (SMOTE) was employed to produce a balanced training set.
Results
Baseline characteristics
The patients undergoing myocardial revascularization without concomitant mitral valve intervention were slightly younger (64.4±11.0 vs. 66.0±10.8 years; P=0.006) and more predominantly male (71% vs. 59%; P<0.001; Table 2). Although this group had a higher incidence of both hyperlipidemia (82% vs. 77%; P=0.030) and a family history of coronary artery disease (38% vs. 27%; P<0.001), there were no significant differences in incidence of diabetes, hypertension, peripheral vascular disease, or stroke.
The patients undergoing revascularization with concomitant mitral valve intervention also had a higher rate of dialysis dependence (7% vs. 4%; P=0.006), chronic lung disease (29% vs. 17%; P<0.001), and prior myocardial infarction (69% vs. 59%; P<0.001). Similarly, this group had a higher rate of New York Heart Association (NYHA) class III (32% vs. 20%; P<0.001) and Class IV heart failure symptoms (46% vs. 36%; P<0.001), as well as prior arrhythmias (27% vs. 15%; P<0.001). Further, the group undergoing revascularization and concomitant mitral valve surgery were also significantly more likely to present in cardiogenic shock (14% vs. 6%; P<0.001) and require an urgent operation (8% vs. 5%; P=0.031).
Patient characteristics according to coronary angiography and echocardiography
The patients undergoing myocardial revascularization alone were more likely to have three-vessel coronary artery disease (69% vs. 61%; P=0.003), while the patients who underwent myocardial revascularization with concomitant mitral valve intervention had a lower pre-operative left ventricular ejection fraction (39.6%±14.6% vs. 48.7%±14.6%; P<0.001). They also experienced a higher rate of complete occlusion of the right coronary artery (39% vs. 28%; P<0.001) and circumflex coronary artery (18% vs. 9%; P<0.001), compared to those undergoing only CABG.
Receiver operating characteristic curve analysis
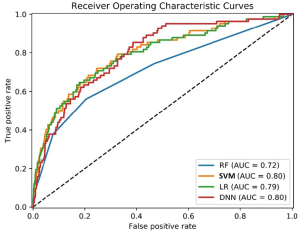
Following training, final models were used to predict class labels for the patients in the test set. The results of these analyses were used to produce receiver operating characteristic curves (Figure 1) for each of the four algorithms under consideration. Three of the models predicted class labels with similar accuracy as demonstrated by their ROC curves, with area under the curve of 0.80, 0.79, and 0.80 for SVM, LR, and DNN, respectively. The RF model produced the ROC curve with the smallest area under the curve of 0.70, and will not be discussed further.
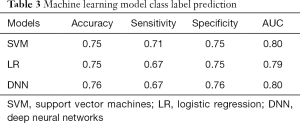
As shown in Table 3, the SVM, LR, and DNN models were able to predict class labels with an accuracy of 0.75, 0.75, and 0.76, respectively. The SVM model had the highest sensitivity of 0.71, followed by LR and DNN with sensitivities of 0.67. Finally, each model displayed similar specificities of 0.75, 0.75, and 0.76 for SVM, LR, and DNN, respectively.
Full table
Feature importance analysis
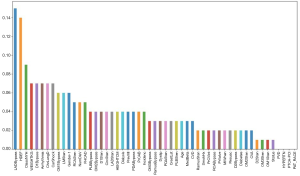
The feature importance analysis suggested most of the STS variables contributed to the identification of clinically significant IMR. The necessity of a bypass graft to the left anterior descending artery, global left ventricular ejection fraction, and NYHA functional class all appeared to be particularly useful in this determination (Figure 2). Interestingly, patient weight and gender, performance of a first diagonal or obtuse marginal bypass graft and the total number of diseased vessels, and the presence of pre-operative arrhythmias, chronic lung disease, cardiogenic shock, left main stenosis, right coronary artery stenosis, and a family history of coronary artery disease all seemed to separate themselves out as being important determinants of classification.
Discussion and conclusion
Machine learning is optimal for the investigation of a wide variety of clinical problems involving predictive modeling (16-18). In comparison to previous approaches, it has the unique ability to actually inform the investigative process by the identification of non-intuitive and otherwise undiscoverable data patterns that predict outcomes for a variety of complex clinical problems (16-18). The machine learning models developed from our single-institution IMR data confirmed a predictive association between isolated STS Database variable patterns and the presence of clinically significant IMR. Using only STS variables as predictors, the AUC achieved by the SVM, LR, and DNN machine learning analyses all demonstrated considerable accuracy in identifying the presence of clinically significant IMR in this coronary revascularization subpopulation. This association was demonstrated despite exclusion of all valvular disease STS Database variables.
To the best of our knowledge, this study is the first to use machine learning to confirm a potentially useful association between isolated STS Database variables and the presence of IMR. This known association may lend considerable predictive utility to these standardized clinical variables in future machine learning modeling to address ongoing controversies in the surgical care of IMR. Even as minimally invasive catheter-based mitral valvular repair strategies are clinically adopted, post-repair recurrence secondary to progressive regional LV remodeling will continue to make these STS Database machine learning models clinically relevant in the management of these patients. The near-universal availability of the STS Database variables from almost every cardiac surgical program in the country makes them particularly attractive in machine learning models. Diagnostic and therapeutic clinical algorithms based upon their use should have immediate and widespread applicability. In light of this ready availability and their proven association with the myocardial, coronary angiographic, demographic, and comorbidity substrate responsible for the inception of IMR, it is safe and reasonable to expect these variables to play a significant role in future modeling of IMR patients.
Future machine learning models incorporating these variables may identify predictive variable combinations that assist clinicians in addressing such controversial issues as the timing of surgical intervention for the various degrees of IMR severity, particularly in that gray-zone of 2–3+ IMR (3-6,35). Further still, machine learning may be used to address the type of cardiac surgical intervention chosen to maximize durability in the amelioration of IMR, which still remains a challenge for cardiac surgeons (36,37). Although the many advantages of repair have made it the most common choice, almost 60% of patients undergoing mitral valve repair for IMR experience recurrence or death by 2 years of follow-up (37,38). Reliable metrics for predicting failure of valve repair in this patient population continue to be of clinical importance. The ability of these STS Database variables to assist in the identification of that clinical substrate that produces IMR, a capability supported by our results, may be particularly applicable. Their inclusion may augment accuracy in future machine learning models directed toward the identification of patients in whom mitral repair, instead of replacement, may deteriorate over time into a simple restoration of this same IMR-inciting substrate. Their addition, for instance, to machine learning models based primarily upon mitral structural morphology and regional contractile injury distribution patterns has considerable potential to assist clinicians in these difficult decisions.
The actual machine learning model parameters developed in this investigation also may be directly applicable in future machine learning investigations. For example, the learned predictive capability of the DNN model developed from our large single-institution investigation can be reused in future modeling to enhance learning performance. With advanced transfer learning techniques, the knowledge gained from our DNN model, which is contained in the nodal weights learned from the institutional STS Database raw variable values of our 7,005 patients, can be transferred to future modeling of other data arrays from new patient cohorts with a reasonable expectation of improved accuracy.
Limitations
This was a retrospective and non-randomized study, thus subjected to inherent selection bias. Even though this is a retrospective analysis, the very nature of the prospective acquisition of mandatory, rigidly structured STS Database variables mitigates many potential biases, including recall, misclassification, self-selection, and differential referral biases. All of the surgical procedures were performed at a single institution, which may impair the extrapolation of our machine learning results to other centers. Further, the supervised machine learning used binary classification based upon the presence of a “clinically significant” degree of IMR. The definition of clinical significance was based solely upon the operative surgeon’s decision regarding the clinical necessity of mitral valve intervention concomitant with revascularization. This decision is influenced by many clinical factors that may have had a variable impact upon the individual surgeons at this institution. Nonetheless, the availability, variable preoperative interval, inconsistent interpretation, dynamic nature of IMR, and resulting wide variability in echocardiographic estimation of degree of IMR made the use of an echocardiographic-based metric of clinically significant IMR even more prohibitive. It is our contention that the operative surgeon had the best overall perspective in judging the clinical significance of their individual patient’s IMR, as reflected in their decision to intervene.
Finally, these STS Database variables characterize only a fraction of the clinical factors associated with the presence of IMR. It was never the goal of this focused investigation to attempt the machine learning predictive modeling of the future aimed at solving current clinical dilemmas. These complex models of the future will include all of the available clinical features that have previously demonstrated potential for inclusion in such predictive modeling. Instead, the simple goal of this investigation was to begin the construction of the foundation of these future all-inclusive models by assessing the association of these STS Database variables with the presence of the clinical substrates that define the IMR patient. The capability of STS Database variables to characterize the clinical, mechanical, phenotypic, and myocardial substrates associated with the occurrence of ischemic MR places it in a prominent position to contribute to the immediate development and clinical implementation of models to predict the patient-specific risk of persistence or post-repair recurrence of ischemic MR.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at http://dx.doi.org/10.21037/jmai-20-50
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jmai-20-50
Peer Review File: Available at http://dx.doi.org/10.21037/jmai-20-50
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jmai-20-50). Three of them report as follows: RJD—Atricure, Inc: Speaker and receives research funding, LivaNova, Inc.: Speaker, Medtronic: Consultant, Edwards Lifesciences: Speaker. AI—Abbott Laboratories: Speaker, Medtronic: Speaker, Abiomed: Speaker. MRM—Medtronic: Consultant. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Washington University School of Medicine Institutional Review Board (IRB Registration # 201905091). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lamas GA, Mitchell GF, Flaker GC, et al. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and Ventricular Enlargement Investigators. Circulation 1997;96:827-33. [Crossref] [PubMed]
- Chaput M, Handschumacher MD, Tournoux F, et al. Mitral leaflet adaptation to ventricular remodeling: Occurrence and adequacy in patients with functional mitral regurgitation. Circulation 2008;118:845-52. [Crossref] [PubMed]
- Enriquez-Sarano M, Schaff HV, Orszulak TA, et al. Congestive heart failure after surgical correction of mitral regurgitation. A long-term study. Circulation 1995;92:2496-503. [Crossref] [PubMed]
- Milano CA, Daneshmand M, Rankin J, et al. Survival Prognosis and Surgical Management of Ischemic Mitral Regurgitation. Ann Thorac Surg 2008;86:735-44. [Crossref] [PubMed]
- Harris KM, Sundt T, Aeppli D, et al. Can late survival of patients with moderate ischemic mitral regurgitation be impacted by intervention on the valve? Ann Thorac Surg 2002;74:1468-75. [Crossref] [PubMed]
- Maltais S, Schaff H, Daly R, et al. Mitral regurgitation surgery in patients with ischemic cardiomyopathy and ischemic mitral regurgitation: Factors that influence survival. J Thorac Cardiovasc Surg 2011;142:995-1001. [Crossref] [PubMed]
- Grigioni F, Enriquez-Sarano M, Zehr K, et al. Long-Term Outcome and Prognostic Implications with Quantitative Doppler Assessment. Circulation 2001;103:1759-64. [Crossref] [PubMed]
- Kron IL, LaPar D, Acker M, et al. 2016 update to The American Association for Thoracic Surgery (AATS) consensus guidelines: Ischemic mitral valve regurgitation. J Thorac Cardiovasc Surg 2017;153:e97-e114. [Crossref] [PubMed]
- Kang DH, Kimg MJ, Kang SJ, et al. Mitral Valve Repair versus Revascularization Alone in the Treatment of Ischemic Mitral Regurgitation. Circulation 2006;114:I499-503. [Crossref] [PubMed]
- Kopjar T, Gasparovic H, Mestres C, et al. Meta-analysis of concomitant mitral valve repair and coronary artery bypass surgery versus isolated coronary artery bypass surgery in patients with moderate ischemic mitral regurgitation. Eur J Cardiothorac Surg 2016;50:212-22. [Crossref] [PubMed]
- Diodato MD, Moon M, Pasque M, et al. Repair of ischemic mitral regurgitation does not increase mortality of improve long-term survival in patients undergoing coronary artery revascularization: A propensity analysis. Ann Thorac Surg 2004;78:794-99. [Crossref] [PubMed]
- Goland S, Czer L, Siegel R, et al. Coronary Revascularization Alone or with Mitral Valve Repair: Outcomes in Patients with Moderate Ischemic Mitral Regurgitation. Tex Heart Inst J 2009;36:416-24. [PubMed]
- Acker MA, Parides MK, Perrault LP, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med 2014;370:23-32. [Crossref] [PubMed]
- Perrault LP, Moskowitz AJ, Kron IL, et al. Optimal surgical management of severe ischemic mitral regurgitation: to repair or to replace? J Thorac Cardiovasc Surg 2012;143:1396-403. [Crossref] [PubMed]
- Gillinov AM, Wierup PN, Blackstone EH, et al. Is repair preferable to replacement for ischemic mitral regurgitation? J Thorac Cardiovasc Surg 2001;122:1125-41. [Crossref] [PubMed]
- Brajer N, Cozzi B, Gao M, et al. Prospective and External Evaluation of a Machine Learning Model to Predict In-Hospital Mortality of Adults at Time of Admission. JAMA Netw Open 2020;3:e1920733 [Crossref] [PubMed]
- Penson A, Camacho N, Zheng Y, et al. Development of Genome-Derived Tumor Type Prediction to Inform Clinical Cancer Care. JAMA Oncol 2019;6:84-91. [Crossref] [PubMed]
- Ting DS, Cheung CY, Lim G, et al. Development and Validation of a Deep Learning System for Diabetic Retinopathy and Related Eye Diseases Using Retinal Images from Multiethnic Populations with Diabetes. JAMA 2017;318:2211-23. [Crossref] [PubMed]
- He S, Fontaine AA, Schwammenthal E, et al. Integrated mechanism for functional mitral regurgitation: leaflet restriction versus coapting force: in vitro studies. Circulation 1997;96:1826-34. [Crossref] [PubMed]
- Otsuji Y, Handschumacher MD, Schwammenthal E, et al. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation 1997;96:1999-2008. [Crossref] [PubMed]
- Ciarka A, Braun J, Delgado V, et al. Predictors of mitral regurgitation recurrence in patients with heart failure undergoing mitral valve annuloplasty. Am J Cardiol 2010;106:395-401. [Crossref] [PubMed]
- Magne J, Pibarot P, Dumesnil JG, et al. Continued global left ventricular remodeling is not the sole mechanism responsible for the late recurrence of ischemic mitral regurgitation after restrictive annuloplasty. J Am Soc Echocardiogr 2009;22:1256-64. [Crossref] [PubMed]
- Lee AP, Acker M, Kubo S, et al. Mechanisms of recurrent functional mitral regurgitation after mitral valve repair in nonischemic dilated cardiomyopathy: importance of distal anterior leaflet tethering. Circulation 2009;119:2606-14. [Crossref] [PubMed]
- Gelsomino S, Lorusso R, De Cicco G, et al. Five-year echocardiographic results of combined undersized mitral ring annuloplasty and coronary artery bypass grafting for chronic ischaemic mitral regurgitation. Eur Heart J 2008;29:231-40. [Crossref] [PubMed]
- Lancaster TS, Kar J, Cupps B, et al. Topographic mapping of left ventricular regional contractile injury in ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2017;154:149-58.e1. [Crossref] [PubMed]
- Cupps BP, Taggar AK, Reynolds LM, et al. Regional myocardial contractile function: multiparametric strain mapping. Interact Cardiovasc Thorac Surg 2010;10:953-7. [Crossref] [PubMed]
- Cupps BP, Bree DR, Wollmuth JR, et al. Myocardial viability mapping by magnetic resonance-based multiparametric systolic strain analysis. Ann Thorac Surg 2008;86:1546-53. [Crossref] [PubMed]
- Ho TK. Random decision forests. In: Proceedings of the International Conference on Document Analysis and Recognition; ICDAR. 1995. doi:
10.1109/ICDAR.1995.598994 . - Cortes C, Vapnik V. Support-Vector Networks. Mach Learn 1995; [Crossref]
- Hosmer D, Lemeshow S, Sturdivant RX. Model-Building Strategies and Methods for Logistic Regression. In: Applied Logistic Regression 2013. doi:
10.1002/0471722146.ch4 . - Bengio Y. Learning deep architectures for AI. Found Trends Mach Learn 2009; [Crossref]
- Han J, Moraga C. The influence of the sigmoid function parameters on the speed of backpropagation learning. In: Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). 1995. doi:
10.1007/3-540-59497-3_175 . - Nair V, Hinton GE. Rectified linear units improve Restricted Boltzmann machines. In: ICML 2010 - Proceedings; 27th International Conference on Machine Learning. 2010.
- Kingma DP, Ba J. Adam: a method for stochastic optimization. CoRR. 2015.abs/1412.6.
- Michler RE, Smith P, Parides M, et al. Two-Year Outcomes of Surgical Treatment of Moderate Ischemic Mitral Regurgitation. N Engl J Med 2016;374:1932-41. [Crossref] [PubMed]
- Kron IL, Hung J, Overbey JR, et al. Predicting recurrent mitral regurgitation after mitral valve repair for severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2015;149:752-61.e1. [Crossref] [PubMed]
- Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N Engl J Med 2016;374:344-53. [Crossref] [PubMed]
- Gammie JS, Sheng S, Griffith BP, et al. Trends in mitral valve surgery in the United States: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2009;87:1431-37. [Crossref] [PubMed]
Cite this article as: MacGregor RM, Kachroo P, Guo A, Schulte L, Cupps BP, Moon MR, Damiano RJ Jr, Damiano M, Burmeister T, Maniar H, Melby SJ, Itoh A, Masood MF, Kotkar K, Pasque MK, Foraker R. Developing a machine learning model using isolated Society of Thoracic Surgeons database variables to predict the presence of clinically-significant ischemic mitral regurgitation. J Med Artif Intell 2021;4:3.


